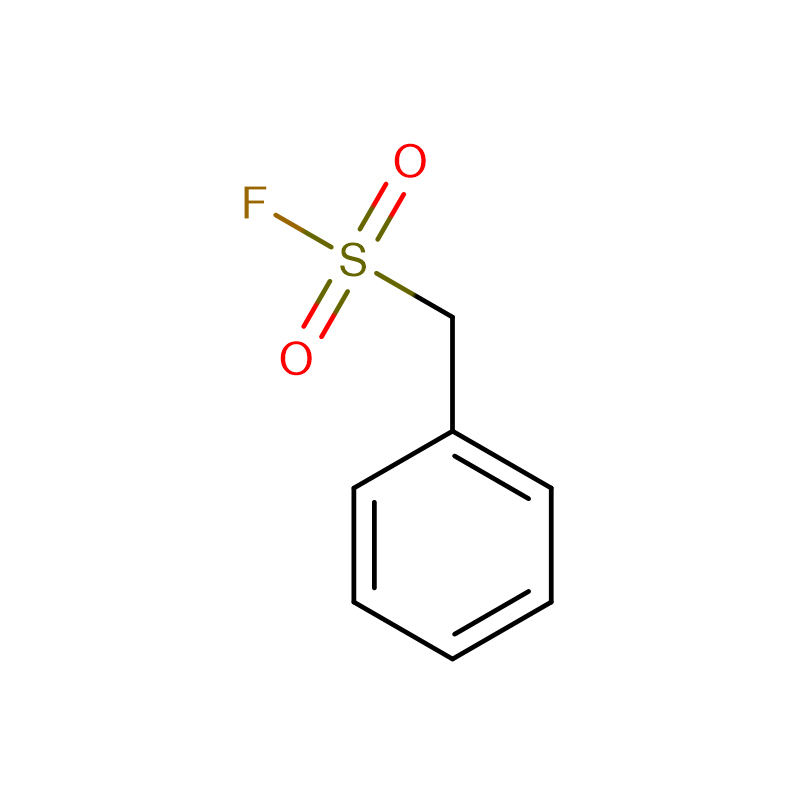
PMSF Cas: 329-98-6 98.0% pulveris crystallini albi Phenylmethanesulfonyl fluoride (PMSF)
| Catalogue Number | XD90250 |
| Product Name | Phenylmethanesulfonyl fluoride (PMSF) |
| CAS | 329-98-6 |
| Formulae hypotheticae | C7H7FO2S |
| M. Pondus | 174.1927 |
| Repono Details | Ambiens |
| Tariff Code harmonized | 29049900 |
Product Specification
| Assay | ≥98.0% HPLC |
| Aspectus | album crystallinum pulveris |
PMSF immedicabile est serine/cysteinum protease inhibitoris vulgo in praeparatione lysatis cellularum adhibitarum.
In studiis vitro: PMSF (2 mM) cumulationis inositolae phosphatae inositolae inhibuit carbacholum inhibuit solum 15%-19% coram Li+.Inhibitio turnoveri phosphoinositidis per PMSF vel uno vel pluribus gradibus sequi phosphoinositidum naufragium est [1].PMSF inhibet acylationem reliquiarum inositol GPI intermediarum in sanguinis rivi T. brucei.PMSF inhibet glycolipidis C formationem, sed non pingue acidum in vitro molestie lacus.PMSF inhibet additionem GPI acylationi et ethanolamine phosphatasi in trypanosomes procyclicis, non autem in cellulis Hela[2].
In studiis vivo: PMSF (0.1 mL/10 g b.wt, ip) producta antinociceptionis, ut patet per augmentum dosis responsivum in %MPE in aestimatione latentium caudae-flick, sed non potuit clarum doses-responsivum motoris inhibitionis producere.Mures, qui intraperitoneam iniectionem PMSF receperunt, effectus cannabinoidei inter antinociceptionem, hypothermia et immobilitatem cum valoribus ED50 86, 224 et 206 mg/kg receperunt.PMSF (30 mg/kg) praetractatio effectus anandamidei in responsionibus caudati (antinociceptionis), locomotoris actuositatis et mobilitatis per 5-ovilem, 10 centuplum et 8-ovile, respective[3] auget.
Animalia experimenta: Male ICR mures pensantes 18 ad 25 g usi sunt in primordio.PMSF oleo sesamo dissolutum et intraperitonealiter in volumine 0.1 mL/10 g b.wt administratum.Semper PMSF 10 minuta administrant ante anandamida vel injectionem vehiculi intravenae.Mures acclimantur ad locum aestimationis pernoctare sine intermissione cibi vel aquae.Sequentes anandamida vel vehiculi administratio intravenosa, unumquodque animal hoc modo aestimatum est: 5 minuta pro latency (antinociceptiva) responsa et 5 ad 15 minuta pro spontanea (motor) actione;vel 5 minuta pro core (rectali) temperationis et anuli Immobilizationis (catalepsy) pro 5 ad 10 minuta.