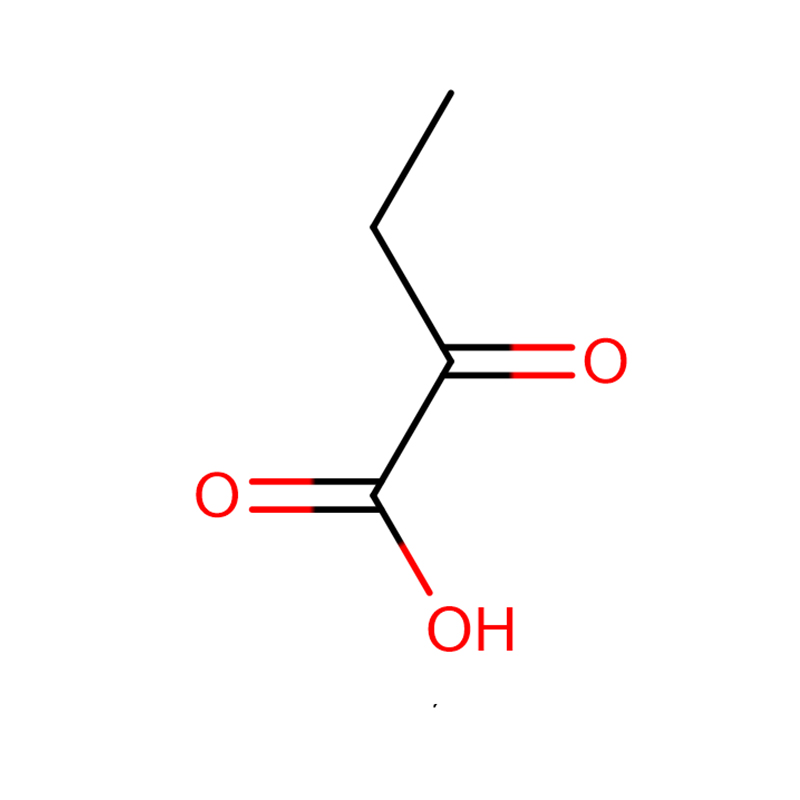
Acidum Oxobutyricum 2-CAS: 600-18-0 subhyalinum
| Catalogue Number | XD90263 |
| Product Name | Acidum Oxobutyricum 2- |
| CAS | 600-18-0 |
| Formulae hypotheticae | C4H6O3 |
| M. Pondus | 102.089 |
| Repono Details | -20 °C |
| Tariff Code harmonized | 2918300090 |
Product Specification
| Density | 1.182 |
| Liquescens punctum | 30-34 °C |
| Ferveret | 84 °C |
| Aspectus | hyalina crustulum |
| Assay | 99% |
1.Optice activum D-2-hydroxybutanoatum est intermedium impedimentum magni ponderis ad medicamenta et poly(2-hydroxybutanoata).Momentum resolutio racemici 2-hydroxybutanoate viridis et desiderabilis alternatio potest esse pro productione hydroxybutanoate D-2-hydroxybutanoate.In hoc opere, D-2-hydroxybutanoate ad attentionem (0.197 M) et alta excessus enantiomeric (99,1%) productus est ab NAD-independens L-lactatum dehydrogenas (L-iLDH) biocatalyticum continens.2-Oxobutanoate, aliud intermedium magni momenti, coaevum ad attentionem (0.193 M).Simplex ion processus permutationis cum macroporosa anion commutationis resinae D301 usus, D-2-hydroxybutanoate a systemate biotransformationis cum magno recuperatione 84.7% separatus est.
2. Asymmetrica synthesis acidi aminoi innaturalis demonstratum est per omega-transaminatam e Vibrio fluviali JS17.Acidum l-2-aminobutyricum cum acido oxobutyrico 2-oxobutyrico et benzylamine summatim cum excessu enantiomericano superiore quam 99% perstringitur.Reactio ostendit grave productum inhibitionis ab benzaldehyde, quae superata est adhibitis reactionis biphasicae systematis ad tollendum opus inhibitorium ab aqueo phase.In reactionem typicam biphasicam (50 mM 2-acidi oxobutyrici, 70 mM benzylaminae et 2.64 U/ml enzyme purgati) utens hexane extractante, conversio acidi 2-oxobutyrici 96% in 5 h pervenit, cum tantum 39% sine conversione impetrata est. producto extractione.